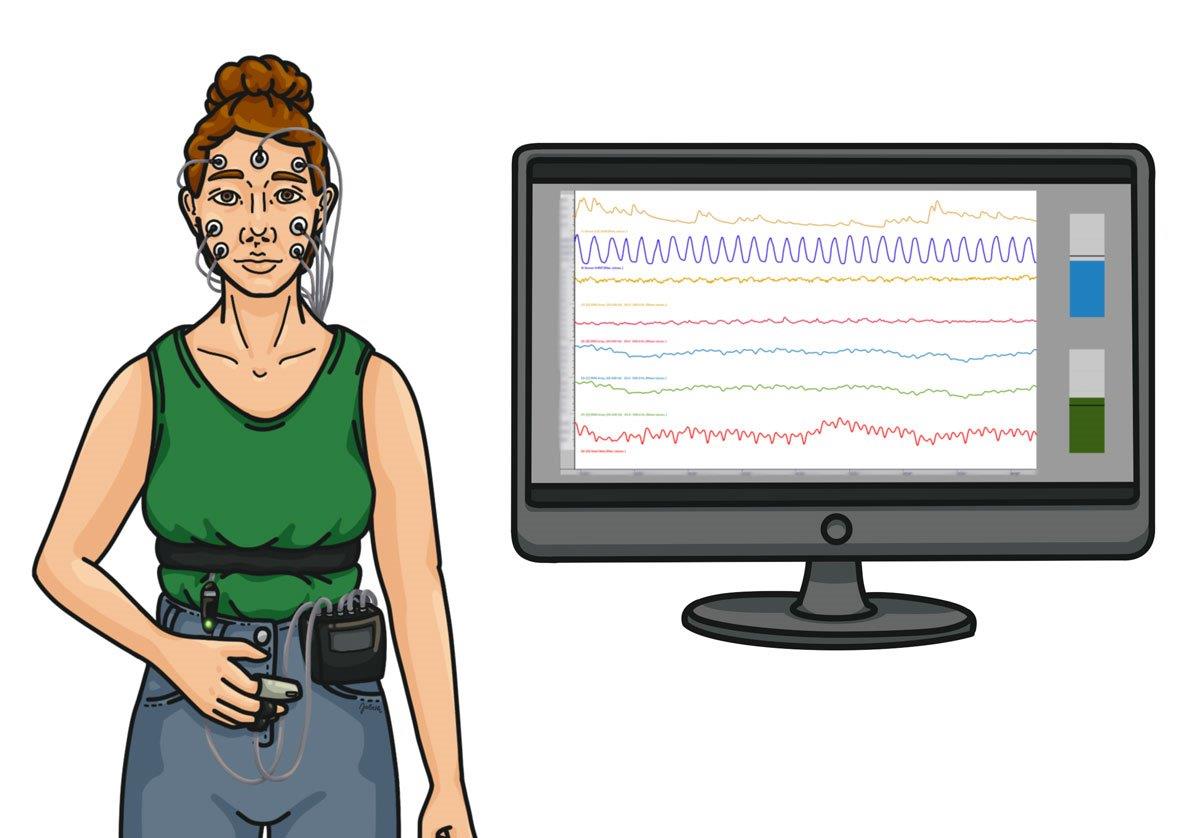
Deutsche Gesellschaft für Biofeedback e.V.

Bio- und Neurofeedback sind innovative, auf empirischer Wissenschaft beruhende Therapiemethoden.
Die Messung von Signalen und eine visuelle oder akustische Rückmeldung machen Vorgänge innerhalb des Körpers (Biofeedback) bzw. des Gehirns (Neurofeedback) in Echtzeit sichtbar. Dadurch können Patientinnen und Patienten lernen, ebendiese Vorgänge aktiv zu steuern.
Die Wirksamkeit der Methoden ist mittlerweile in vielfältigen Einsatzgebieten belegt. Bei der Behandlung von Schmerzsyndromen, Erkrankungen des Herz-Kreislauf-Systems, psychischen und psychosomatischen Beschwerden oder ADHS sowie auch im Bereich des Coachings oder sportlichen Leistungs-Trainings werden Bio- und Neurofeedback erfolgreich angewendet.
Aktuelle Termine
Online Qualitätszirkel Biofeedback/Neurofeedback:Neurofeedback Arbeitskreis: 1. Dienstag im Monat 19:00 Uhr, alle 2 Monate, nächster Termin 4. Juni 24, 19:00 Uhr. QZ Biofeedback:5.Juni , 21.August, 6. November jeweils um 19:30 bis 21:00 Uhr.
Nächster Bibo-Technik-Termin wird in Kürze bekannt gegeben, die Gruppe freut sich auch über neue Mitglieder, gerne können Sie sich hierfür an das Sekretariat, sekretariat@dgbfb.de wenden.
Alle unsere Workshops finden Sie in den Fortbildungen der DGBfb.
Auf Wunsch unserer Mitglieder haben wir zahlreiche Kurse auf das Hybrid Format umgestellt. Schauen Sie sich die Fortbildungsliste an. Aber natürlich gibt es auch weiterhin Kurse in Präsenz.
- Feedback der langsamen kortikalen Potentiale, 4. Mai 2024, 10:00 – 18:00 Uhr, Universitätsklinikum Tübingen
- Biofeedback und Neurofeedback bei Schlafstörungen, 8. + 9. Juni 2024, 10:00 – 17:00 Schön Klinik Roseneck, Prien am Chiemsee
- Herzratenvariabilitäts-Biofeedback 20. + 21.Juli 2024, 10:00 – 17:00 Uhr, Karlsruhe
Die Deutsche Gesellschaft für Biofeedback (DGBfb e.V.)
Die DGBfb ist die einzige unabhängige Fachgesellschaft für die Methode des Bio- und Neurofeedbacks in Deutschland. Sie hat etwa 525 Mitglieder, insbesondere aus den Bereichen Psychologie, Psychotherapie, Medizin und weiteren Gesundheitsberufen.
Sie hat sich folgende Ziele gesetzt:
- Verbreitung von Information über Biofeedback für Patienten und Behandler
- Förderung von Biofeedback in Praxis, Lehre und Forschung
- Angebot einer qualifizierten Aus- und Fortbildung in der Biofeedback-Therapie
- Internationale Zusammenarbeit mit anderen Biofeedback-Gesellschaften